OUR PIPELINE
Veterinary infections![]()
Animal models have been used to test applications of DIBI against a wide variety of infections including skin infections, eye, ear and pulmonary infections as well as systemic infections. Fe Pharmaceuticals intends to focus its resources on the development of DIBI and related compounds for human health applications and would welcome a partner to undertake development of animal health products based on our polymer anti-infective, anti-inflammatory platform.

Veterinary clinical development of DIBI eardrops has proceeded through Phase 2 in a study comparing DIBI plus a steroid (dexamethasone) to a standard of care product, Surolan® which is a combination of antibiotic, antifungal, and anti-inflammatory medications: polymyxin B, miconazole, and prednisolone.
DIBI otic toxicity testing (Phase 1) was completed in healthy beagles.
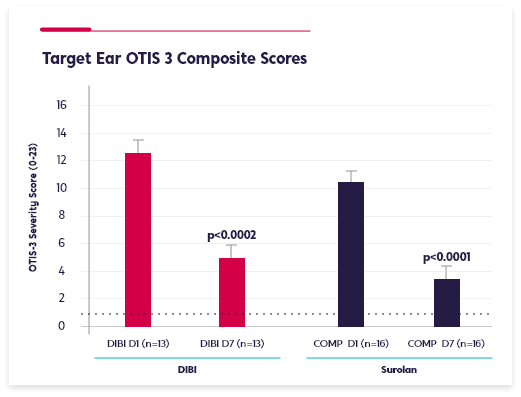
A double-blind Phase 2 clinical study in otitis externa in in-clinic canine patients compared DIBI + dexamethasone to Surolan® (includes prednisolone). Each was administered twice per day for 7 days. 29 dogs (DIBI n=13; Surolan® n=16) satisfied the criteria for data analysis.
DIBI was equivalent to Surolan® in reducing the clinical severity of otitis external.
66 bacterial isolates were recovered from 28 target ears. Both groups had significant within-group clinical improvement (p<0.001) over time and equivalent between group improvement over time.
