Development Pipeline
Fe Pharmaceuticals is developing a pipeline of indications for DIBI in serious diseases exacerbated by iron dysregulation. These are diseases where our iron binding polymer platform can provide a unique solution. Our current focus areas include infectious disease, including antimicrobial resistant (AMR) infections, and sepsis, as well as inflammation and cancer.
Focusing on treatment for:
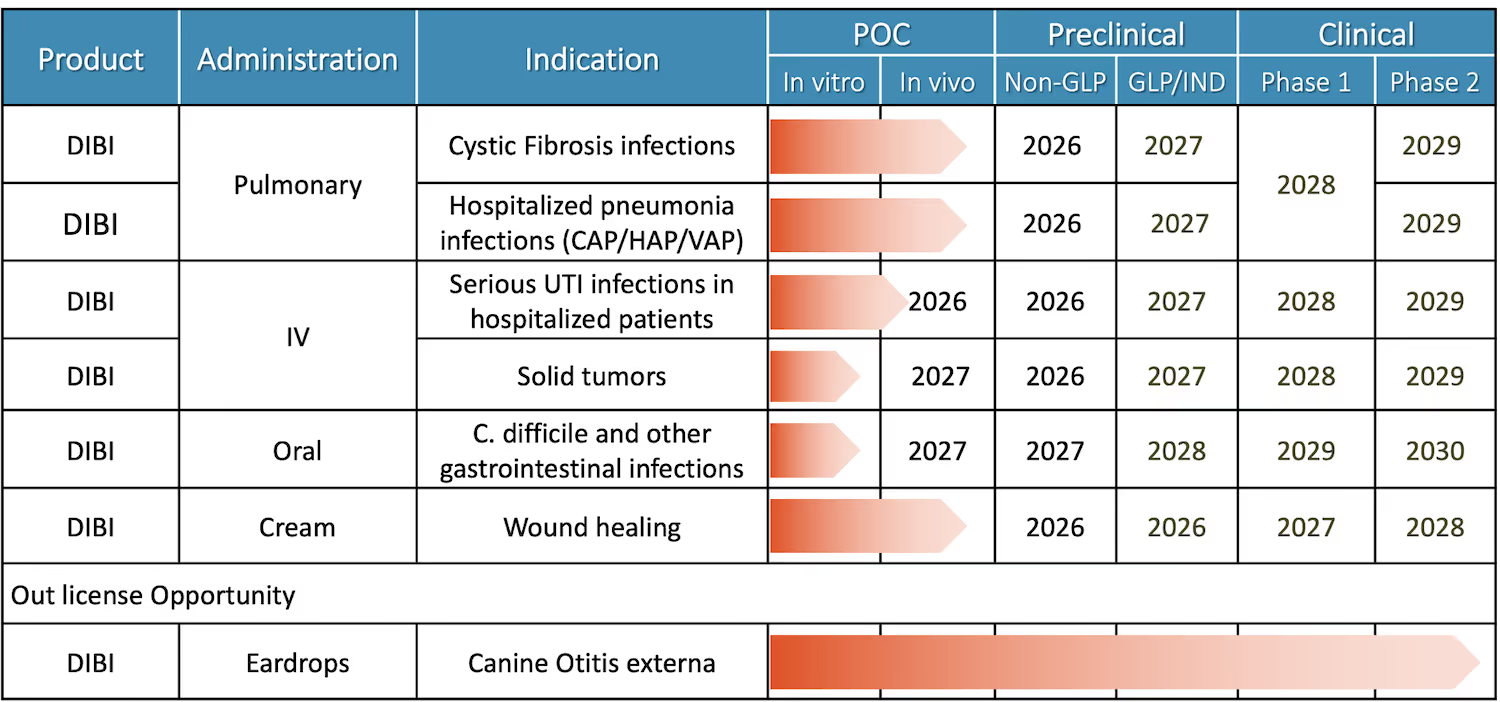
DIBI Applications in Infectious Disease, Oncology, and Veterinary Medicine
Fe Pharmaceuticals’ pipeline includes inhaled DIBI in development for respiratory infections in Cystic Fibrosis patients and hospitalized pneumonia patients, IV DIBI for complicated urinary tract infections and potentially for cancer, and future programs for oral DIBI to treat severe GI (gastrointestinal) infections and a topical cream of DIBI to treat serious skin and soft tissue infections and promote wound healing. A veterinary indication where DIBI was equivalent to standard of care for otitis externa in a Phase 2 study in dogs is available for licensing.
Meeting Serious Threats
Antimicrobial Resistant (AMR) Infections
The re-emergence of infections as a growing health threat and serious cause of mortality is due to the rise of antimicrobial resistant bacterial infections. Bacterial AMR caused an estimated 1.27 million deaths worldwide in 2019 and was associated with 4.95 million deaths (Murray et al, Lancet, 2022). In 2019, the WHO projected that AMR infections would cause 10 million yearly deaths by 2050 surpassing annual deaths from all cancers (O’Neill, 2016). Because exposure to antimicrobial agents drives the emergence of resistance, the standard of care has become "stewardship" of new antibiotics, reserving them only for last line use.
Sepsis
Serious infections in hospitalized patients often lead to sepsis, the leading cause of death in hospitals. There are currently no approved products to treat sepsis, despite affecting nearly 49 million people worldwide each year, killing an estimated 8 million (Rudd et al, Lancet, 2020), and being the most common killer of children, taking more than 3.4 million each year. In the US in 2014, sepsis affected and estimated 1.7 million patients annually resulting in 270,000 deaths (Rhee, et al, JAMA, 2017). 1 in 3 patients who die in US hospitals have sepsis. COVID-19 only made the situation worse.
.webp)
DIBI Treats Serious and Drug Resistant Infections Including Those That Lead to Sepsis
The intersection of these problems is large and growing. Four specific infection categories lead to 82% of all sepsis. An ideal solution would be a very broad-spectrum agent immune to the development of resistance, the exact profile of DIBI. Because resistance cannot emerge, there is no need for antibiotic-style stewardship of DIBI, and DIBI can treat these infections early and prevent or reduce sepsis and its immune system dysregulation that leads to morbidity and mortality.
Infection
Respiratory Infection
- Lower respiratory infection (pneumonia) is the world’s most deadly communicable disease, ranked as the 4th leading cause of death overall; in 2019 (pre-COVID) it claimed 2.6 million lives (WHO, Dec 2020).
- Community acquired pneumonia (CAP) is the second most common cause of hospitalization; ~2-3 million hospitalizations / year US.
- CAP pathogens and the immune overreaction that can develop are iron dependent.
- Pneumonia is the leading precursor infection to sepsis (CDC: 35% of sepsis cases arise from pneumonia).
Ample evidence DIBI will be effective:
- DIBI reduced levels of Pseudomonas aeruginosa bacteria in the lungs of mice in a model of fibrosis.
- DIBI reduced infection in CAP animal models.
- DIBI synergized with standard of care (SOC) antibiotics in CAP models.
- DIBI dampened excess cytokine expression in lung macrophages.
- DIBI reduced excess inflammatory cytokine concentrations in lungs induced by bacterial antigens and reduced lung damage in a mouse model.
- DIBI reduced cytokine overexpression (“cytokine storm”) in sepsis models and reduced cardiovascular damage.
- DIBI improved survival in sepsis models and synergized with an antibiotic.
.avif)
.webp)
Urinary Tract Infection (UTI)
- UTIs are 2nd leading cause of sepsis with multidrug resistance common – may provide window to decreased sepsis.
- UTIs are extremely common in hospitalized patients: 2,837,385 US hospital discharges were made with a UTI code, 500,400 were nCAcUTI (non-catheter complicated UTI) and 126,120 were catheter-associated UTI (CAUTI); >626 000 hospital admissions were made with a cUTI.
Where DIBI can help
- UTIs are highly dependent on iron. During urinary tract infections, humans secrete the protein siderocalin to block bacterial iron uptake.
- Accumulation and residence time of DIBI in kidney seems ideal to block iron in infections.
- Anti-inflammatory properties may help avoid/reduce kidney damage.
Skin and Soft Tissue Infection
- Strong literature evidence that iron levels are correlated with skin and soft tissue infections.
- Diabetic ulcers are a very significant and growing medical need.
- Topical application of an iron chelator showed better reduction of diabetic wounds in a Phase 1/2 study (Lyons et al, 2006).
Ample evidence DIBI will be effective
- DIBI inhibits MRSA and Pseudomonas (low MICs; minimum inhibitory concentrations), the key pathogens in diabetic ulcers.
- Delivery of 2% DIBI cream to skin in animal models reduces infection CFUs caused by MRSA.
- Topical DIBI synergizes with antibiotic (fusidic acid) in MRSA skin infection model.
- Topical DIBI inhibits inflammation in MRSA skin infection model. Intraperitoneal DIBI similarly inhibits inflammation and cytokine expression in lung macrophages.
- In vitro DIBI inhibits biofilm formation (a major source of pathogenicity and antibiotic resistance).
- Direct delivery to the wound by a cream or ointment or wound dressing is well accepted by these patients.
- Once established in a skin infection indication, burns, diabetic ulcers, sickle cell ulcers, pressure ulcers, and other chronic wounds expand market upside.
.webp)
Cancer
Addressing The Problem
Cancer cells need more iron, continuously supplied, due to their high growth rates. Anti-cancer iron chelators have been sought for years (Torti, S., Torti, F. Iron and cancer: more ore to be mined. Nat Rev Cancer 13, 342–355 (2013). https://doi.org/10.1038/nrc3495 ). Epidemiological evidence links increased body iron stores to increased cancer risk. High intake of dietary iron is associated with an increased risk for some cancers, particularly colorectal cancer. Hereditary haemochromatosis, a genetic disease that leads to excess iron accumulation, is associated with increased cancer risk.
Iron regulates crucial signaling pathways in tumors, including the hypoxia-inducible factor (HIF) and WNT pathways. The expression of ferroportin, hepcidin, transferrin receptor 1(TFR1), haemochromatosis (HFE) and other genes involved in iron metabolism is linked to the prognosis of patients with breast cancer.
Many types of cancer cells reprogram iron metabolism in ways that result in increased iron uptake. They upregulate proteins that are involved in iron uptake, such as transferrin receptor 1 (TFR1), and decrease the expression of iron efflux proteins, such as ferroportin.
Fe Pharmaceuticals' Research
Iron management is a valid target for cancer therapy.
DIBI alone kills breast cancer cell lines in a dose-dependent manner while having a very low cytotoxicity for normal cells.



DIBI acts as a chemosensitizer – greatly enhancing cancer chemotherapeutic agents. DIBI was synergistic with cisplatin, doxorubicin, and cyclophosphamide.



The next steps for IV DIBI in oncology development are extensive animal studies in different cancer xenograft models.

